-
About
- About Listly
- Community & Support
- Howto
- Chrome Extension
- Bookmarklet
- WordPress Plugin
- Listly Premium
- Privacy
- Terms
- DMCA Copyright
- © 2010-2025 Boomy Labs

Listly by Kajal Panda
Chemistry basic topics for high school 9, 10, 11, 12 grade or class education. These topics published on this page are helpful for different types of examination.
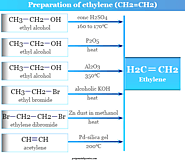
Ethylene formula, structure, production, reaction, uses and properties. Preparation from ethyl alcohol or chloride, hydrogenation and addition of ethylene
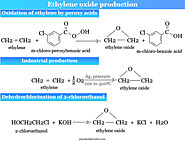
Ethylene oxide molecular formula, structure, production, reduction, dimerization reaction, properties and uses for preparation ethylene glycol, cellosolve
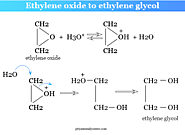
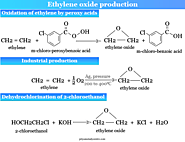
Ethylene oxide to ethylene glycol is converted in a dilute acid solution. It also produced mono-ethers with alcohol in presence of a small amount of acid. The mechanism of the reaction in acid media follows the steps given in the picture above.
Ethylene glycol or ethane-1,2-diol is a colourless viscous liquid with the formula (CH2OH)2. It is missable in all proportions with water and alcohol but insoluble in ether. The prefix glyc in ethylene glycol indicates that the organic compound has a sweet taste. It is widely used as a solvent and antifreezing agent... more about Ethylene glycol properties

Germanium facts, element, properties, isotopes, uses and found on periodic table, production and tetrafluoride, tetrachloride, oxide compounds of germanium
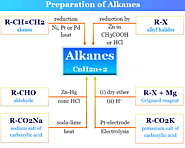
Alkanes or paraffin general molecular formula, structure, properties, uses and methods of preparation in organic chemistry, paraffin wax production
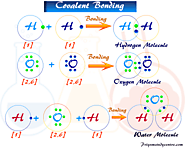
Covalent bond and compound definition, types, properties, examples, Lewis structure, octet rule for bonding single, double, triple covalent bonds
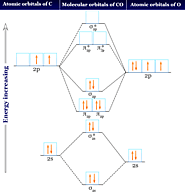
Carbon monoxide facts, formula, uses, bonding, Properties. Hybridization, molecular orbital diagram, production and chemical reaction carbon monoxide
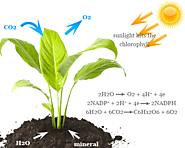
Carbon dioxide in atmosphere, molecular formula, structure, facts, uses of liquid or gas molecule. Chemical reaction and physical properties of CO2
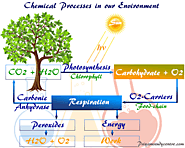
Environmental chemistry or science, abiotic biotic energy components, ecosystem, theory responsible in origin of life, our environment conservation
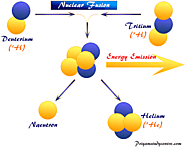
Nuclear fusion definition, facts, uses, equation and working process, fusion energy from mass defect, source of steller energy in sun or star
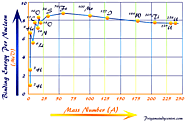
Nuclear binding energy and mass defect definition, formula, curve to calculate average binding energy per nucleon in chemistry, helium atom mass defect
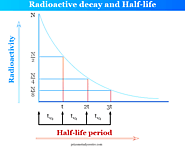
Radioactive decay formula and half-life period, calculation, and application in carbon-14 dating or age of rock, decay constant and mean life in chemistry
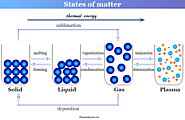
States of matter in physics or chemistry definition, types and characteristics aggregation of molecules in solid, liquid, gaseous and plasma state of matter

Free online quizzes or multiple choice question answer (MCQ) on chemistry and biology for general competitive examinations to test the students knowledge
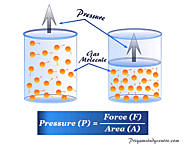
What is Pressure in Science? Definition, formula and measurement, types, unit and dimension of the atmospheric pressure of gas molecules in chemistry
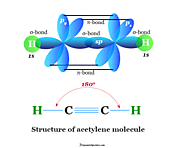
Organic compound definition, types, history and examples in chemistry, classification of organic substances, aliphatic, aromatic and heterocyclic compounds
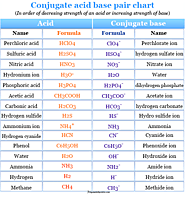
Acids and bases definition, concept, theory and examples Arrhenius and protonic theories or concepts, properties and uses of acid and base in chemistry
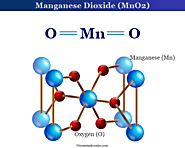
Manganese Dioxide or pyrolusite chemical formula, structure, oxidizing properties. Uses of manganese dioxide in dry cells and chemical industry
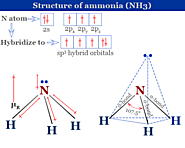
Ammonia formula, properties, structure, production by Haber process and uses in industry, agriculture, household. Chemical reaction and effects of ammonia
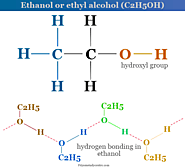
Ethanol or ethyl alcohol formula, production from ethylene and fermentation process, structure, properties, uses in alcoholic drinks, fuel and solvent
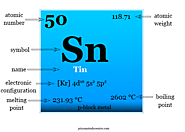
Tin element facts, symbol, chemical properties, uses and found on the periodic table. Occurrence, isotopes and hydride, halide, oxide compounds of tin
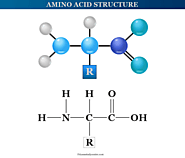
Amino acids definition, formula, structure and properties. Nonessential, essential, conditional types of acids, examples of acidic basic neutral amino acid
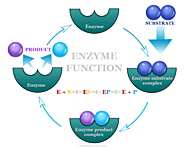
Enzymes definition, function, classification, activity factors and mechanism of catalysis reactions. Examples of inhibitors and types of cofactors in enzyme